Obesity is a complex, multifactorial disease involving an excessive accumulation of body fat that, in time, increases the risk of heart, liver, and kidney diseases, type 2 diabetes, hypertension and certain cancers.
Globally around 14%, and in the US 2 in 5 adults, are obese; obesity is the fifth leading risk for global deaths contributing to at least 2.8 million deaths annually.
Metabolic dysfunction-associated steatotic liver disease (MASLD) is the hepatic manifestation of obesity and the most common form of liver disease affecting nearly 30% of the US population. The prognosis for simple liver steatosis is relatively benign; however, chronic inflammation and progressive fibrosis may lead to metabolic dysfunction-associated steatohepatitis (MASH) and later to cirrhosis and hepatocellular carcinoma. There is currently only one established treatment to inhibit progression from MASLD to MASH that has been approved by the FDA.
Cardiovascular diseases (CVDs) are the number one cause of death globally. In the United States, about 659,000 people die from CVDs each year, which constitutes one in every four deaths. CVDs costs the United States about $363 billion each year, which includes the cost of health care services, medicines, and lost productivity due to death. Most CVDs are caused by risk factors such as tobacco use, physical inactivity, and obesity.
Despite the increase in the number of cases in the past several decades, the underlying molecular mechanisms of cardiac and hepatic metabolic diseases pathogenesis remain poorly understood, thereby preventing the development of effective diagnostic tools and pharmacotherapies as well as preventive strategies.
Research
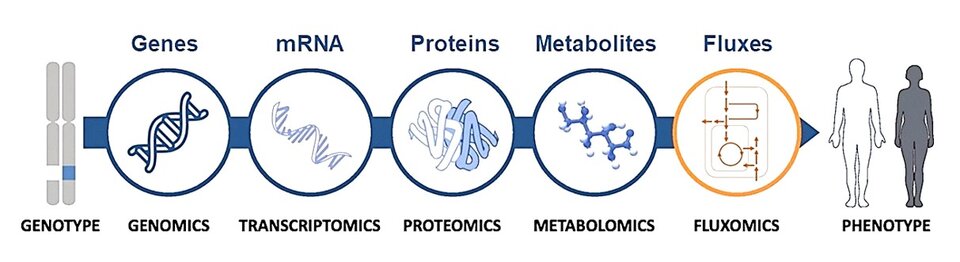
The long-term goal of the Bednarski Lab is to identify metabolic pathways that control the progression of metabolic disorders so that these processes can be targeted in the clinic for disease prevention, diagnosis, or treatment. We use state-of-the-art genomic, transcriptomic, proteomic, metabolomic, and stable isotope tracer methods to establish mechanisms of metabolic disease pathogenesis. In addition to in vitro experiments with primary hepatocytes and cardiomyocyte cell lines, we also use mice with tissue-specific metabolic gene alterations. The in vivo studies involve implementation of the newly developed 13C metabolic flux analysis (MFA) technique, allowing for integrated multi-organ comprehensive quantification of metabolic fluxes.
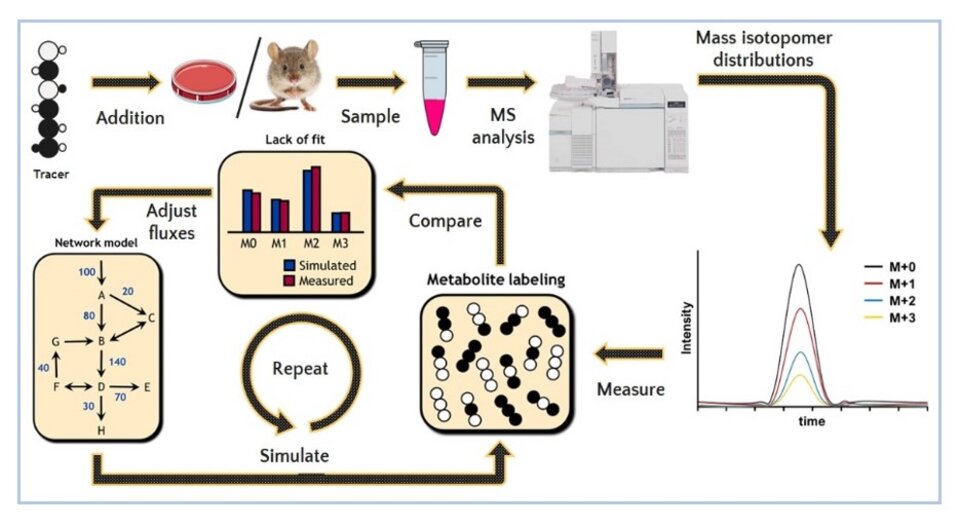
MFA workflow: a stable isotope or radioactive tracer is introduced into the system being studied. Samples are collected, typically cells and media from an in vitro system or plasma and tissues from an in vivo system. Metabolic enrichment is measured using mass spectrometry. A metabolic network is created based on biochemical equations in a specialized flux software package. Metabolite labeling is simulated for the metabolic network based on the initial guesses, which is then compared to the measured enrichment patterns. Fluxes are readjusted and the process is reiterated until reaching the best fit solution, meaning the simulated labeling matches the measured labeling. The end product of metabolic flux analysis is a network map indicating the fluxes through the system being studied.
Objectives
- Determine the role of aberrant polyunsaturated fatty acid metabolism in development of lipotoxic cardiomyopathy.
The working hypothesis is that restoration of proper membrane composition will result in the mitigation of cardiometabolic stress and heart dysfunction. - Identify the lipid metabolism pathways associated with diabetic cardiomyopathy pathogenesis.
The working hypothesis is that adjusting fatty acid transport, synthesis, and utilization will alleviate lipotoxic effects and inhibit cardiac steatosis and inflammation. - Ascertain the role of pyruvate metabolism in the progression of steatotic liver disease.
The working hypothesis is that normalization in pyruvate exchange rate between liver and peripheral tissues will contribute to balancing redox state and alleviating oxidative stress leading to improvement of hepatic function. - Characterize the role of acyl-CoA metabolism in steatotic liver disease pathogenesis.
The working hypothesis is that alteration in acyl-CoA synthesis and transport will alleviate metabolic stress and liver disfunction. - Establish if impaired calcium flux and endoplasmic reticulum stress contribute to pancreatic dysfunction and type 2 diabetes advancement.
The working hypothesis is that enhancing the ability of endoplasmic reticulum to sequester calcium will normalize mitochondrial metabolism and reduce pancreatic α- and β-cell lipotoxicity.
Our central hypothesis is that identifying and targeting processes connected with aberrant lipid metabolism will result in the alleviation of lipotoxic effects and the mitigation of metabolic dysfunction. We expect to uncover druggable nodes that can be modulated in vivo with low risk of side effects.
Additionally, we are working on two highly innovative pilot projects:
- Harnessing the potential of epigenetics, we aim to uncover the mechanism behind "healthy obesity" using hibernating 13-lined ground squirrels as a novel model organism.
- Our goal is to assess the impact of Maca (Lepidium meyenii) supplementation on hypothyroidism pathogenesis in vivo.
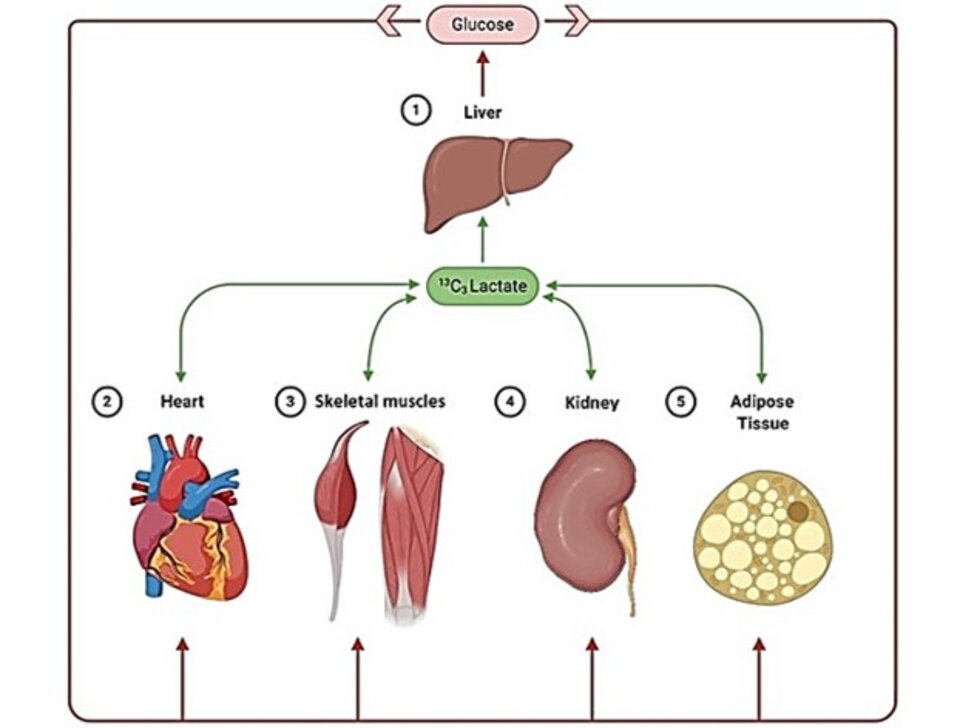
Simultaneous in vivo multi-organ fluxomics in obese mice.
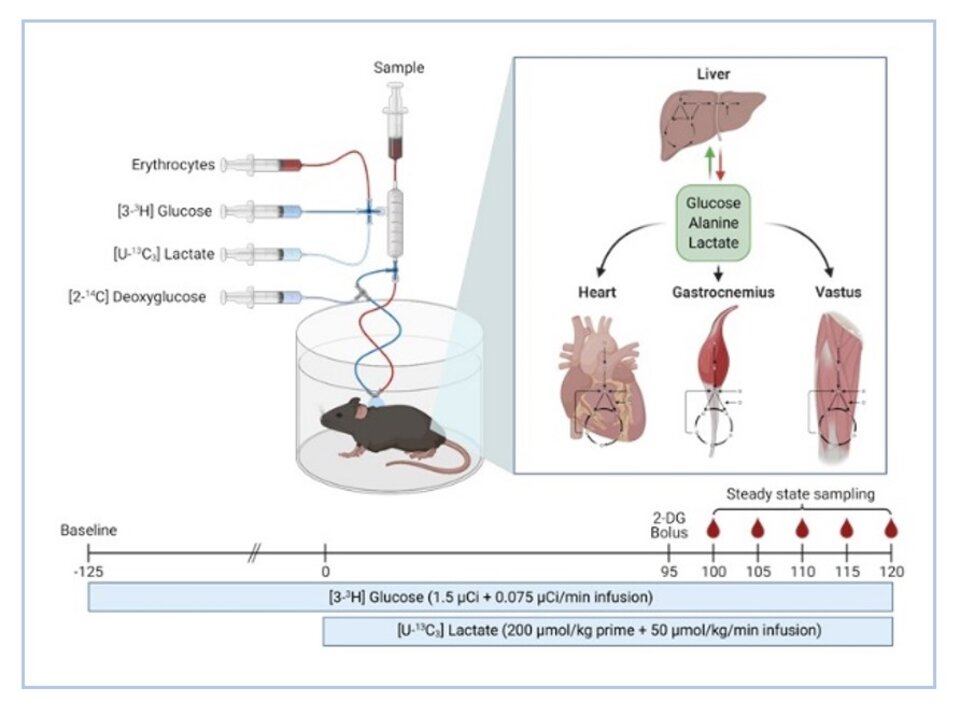
Protocol for multi-tissue MFA in conscious, unrestrained mice using a dual catheter system.
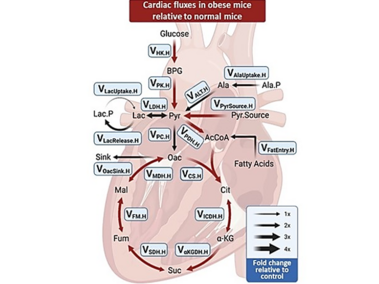
Flux map showing cardiac pathways modeled in obese mice.
Red arrow indicates increased fluxes relative to normal animals.
Lab Members

Tomasz Bednarski
Assistant Professor
tomasz.k.bednarski@unl.edu
Bio
Dr. Bednarski's PhD studies at Nencki Institute of Experimental Biology PAS in Warsaw, Poland, focused on the role of long-chain fatty acid metabolism in different models of left ventricular hypertrophy, resulting in several highly cited publications, numerous conference proceedings, domestic and EU scientific grants and scholarships, and an international patent. Through this research, he demonstrated for the first time that activation of lipogenesis and fatty acid β-oxidation pathways relate to the development of physiological left ventricular hypertrophy and may be one of the adaptive mechanisms to endurance training. He also discovered that the excessive accumulation of triglycerides in cardiac muscle, which is associated with pathological left ventricular hypertrophy, is generated by impairment of the lipolytic process. Part of this research was funded by his pre-doctoral fellowship from the National Science Centre in Poland.
Outside of his scientific work, he devoted himself to science outreach as re-elected chair of the student council, organizer of science popularization events and conferences, and tutor to graduate students.
His commitment to academic and personal excellence led him to postdoctoral training at Vanderbilt University. Throughout his postdoctoral training, he has been at the forefront of the field of fluxomics, which is emerging as an extremely promising tool in the prevention, diagnosis, and treatment of metabolic diseases. His project focused on the application of in vivo 13C metabolic flux analysis and metabolomics profiling to identify liver phenotypes that accelerate the transition from simple liver steatosis to the much more severe steatohepatitis, and to assess in vivo responses to pharmacologic and genetic interventions designed to inhibit this transition. He demonstrated that during chronic, Western diet-induced obesity, pharmacological activation of sarco/endoplasmic reticulum Ca2+-ATPase (SERCA) significantly inhibits NASH progression. He linked these phenotype improvements to the normalization of pyruvate cycling, alleviation of redox imbalance between liver and peripheral tissues, and increase in hepatic ω-3 polyunsaturated fatty acid content. These findings resulted in several poster and oral presentations at ADA, ASBMB, and FASEB conferences as well as multiple publications. For his project assessing liver X receptor (LXR) activation as a strategy to inhibit NASH pathogenesis, he obtained independent financing from the Vanderbilt Diabetes Research and Training Center. He also collaborated with peers to develop a method allowing for simultaneous in vivo multi-organ metabolic flux analysis. This newly developed, powerful tool allows for whole-body assessment of impaired metabolic fluxes, leading to a better understanding of metabolic disorders.
Dr. Bednarski actively participates in the review process of articles for journals specializing in diabetes, hepatology, and lipid metabolism, as well as grants for UNL's Research Council. In addition to his research and service, he contributes to enhancing student success through teaching and mentoring.

Anne Bader
Temporary Research Assistant
abader8@unl.edu
Anne graduated from Grinnell College in 2024 with a BA in Biology and concentrations in Statistics and Neuroscience.
As a neuroinformatics intern at the Michigan Neuroscience Institute, University of Michigan, she completed an independent project conducting a meta-analysis examining changes in gene expression within the anterior cingulate cortex in rats and mice that underwent chronic pain treatment. She presented the results to faculty and fellow lab members at the University of Michigan and at a Grinnell College Biology department seminar.
As a research assistant at Grinnell College within the statistics department, she developed R tutorials for introductory statistics students, beta tested statistics learning materials, and coded in R for data visualization. As an undergraduate at Grinnell College, her research projects included exploring the effects of overexpressing an acid-resistance associated gene in Bacillus subtilis, and observing the localization and dynamics of a protein involved in the anaphase promoting complex in Xenopus embryos using confocal microscopy and Fluorescence Recovery After Photobleaching (FRAP).
Former Lab Members

| Yousuf Al-Farqani Yousuf's work with the Bednarski Lab included investigating cardiomyocyte phospholipid remodeling in obesity and type 2 diabetes. He is now working as a Research Assistant II at MD Anderson Cancer Center in Houston, Texas, which has been consistently rated the nation's #1 hospital for cancer care. |

| Sylwia Miekus Sylwia worked with the Bednarski Lab from 2024-2025 as a Graduate Research Assistant. |

| Joshua Miller Josh was working on his own independent research project through UCARE program over the 2024-2025 academic year investigating the effect of obesity on satellite cell differentiation in mice. He contributed to one original and one review paper. In addition, he included his findings in the honors thesis and graduated With Highest Distinction. Josh was admitted to UNMC and is currently pursuing a medical career. |

| Adam Olichwier Adam helped to establish the Bednarski lab by setting up new equipment and developing methodology. Scientifically, he was leading two main projects, one that focused on the epigenetic control of the process of hibernation and the second investigating the impact of obesity on the satellite cells metabolism. He is now working in the Department of Biopharmacy and Radiopharmacy at the Medical University of Bialystok in Poland. |
Selected Publications
2024
- Pharmacological SERCA activation limits diet-induced steatohepatitis and restores liver metabolic function in mice.
Bednarski TK, Rahim M, Hasenour CM, Banerjee DR, Trenary IA, Wasserman DH, Young JD
Journal of Lipid Research, 2024; 65 (6): 100558. DOI: 10.1016/j.jlr.2024.100558.
2022
- Alterations of lipid metabolism in the heart in spontaneously hypertensive rats precedes left ventricular hypertrophy and cardiac dysfunction.
TK Bednarski, MK Duda, P Dobrzyn
Cells 11(19), 3032 1 2022; DOI: 10.3390/cells11193032
2021
- Multitissue 2H/13C flux analysis reveals reciprocal upregulation of renal gluconeogenesis in hepatic PEPCK-C–knockout mice.
Rahim M, Hasenour CM, Bednarski TK, Hughey CC, Wasserman DH, Young JD
JCI Insight, 2021, 6 (12), e149278; DOI: 10.1172/jcl.insight.149278 - In vivo 2H/13C flux analysis in metabolism research
Bednarski TK, Rahim M, Young JD
Current Opinion in Biotechnology 71, 1-8 8; DOI: 10.1013/j.copbio.2021.04.005
2020
- Vitamin E does not prevent Western diet-induced NASH progression and increases metabolic flux dysregulation in mice
Hasenour CM, Kennedy AJ, Bednarski T, Trenary IA, Eudy BJ, da Silva RP, Boyd KL, Young JD
Journal of Lipid Research, 2020, 61 (5), pp. 707–721; DOI: 10.1194/jlr.RA119000183
2016
- Stearoyl-CoA desaturase 1 deficiency reduces lipid accumulation in the heart by activating lipolysis independently of peroxisome proliferator-activated receptor α
Bednarski T, Olichwier A, Opasinska A, Pyrkowska A, Gan AM, Ntambi JM, Dobrzyn P
Biochimica et Biophysica Acta - Molecular and Cell Biology of Lipids, 2016, 1861 (12), pp. 2029–2037; DOI: 10.1016/j.bbalip.2016.10.005 - Regulation of cardiac metabolism and function by lipogenic factors [Białkowe czynniki lipogenne - Rola w regulacji metabolizmu i funkcji miȩśnia sercowego]
Bednarski T, Pyrkowska A, Opasinska A, Dobrzyn P
Postepy Higieny i Medycyny Doswiadczalnej, 2016, 70, pp. 644–653; DOI: 10.5604/17322693.1206541
2015
- Metabolic reprogramming of the heart through stearoyl-CoA desaturase
Dobrzyn P, Bednarski T, Dobrzyn A
Progress in Lipid Research, 2015, 57, pp. 1–12; DOI: 10.1016/j.plipres.2014.11.003
2013
- Expression of lipogenic genes is upregulated in the heart with exercise training-induced but not pressure overload-induced left ventricular hypertrophy
Dobrzyn P, Pyrkowska A, Duda MK, Bednarski T, Maczewski M, Langfort J, Dobrzyn A
American journal of physiology. Endocrinology and metabolism, 2013, 304 (12), E1348–1358; DOI: 10.1152/ajpendo.00603.2012
Patents
- Method for the early diagnosis of a pre-diabetic state and type 2 diabetes
Dobrzyn P, Dobrzyn A, Kozinski K, Bednarski T; US-10222384-B2
Grants
2024
- ARD Strategic Funding "The impact of Maca (Lepidium meyenii) supplementation on hypothyroidism pathogenesis in vivo."
- CEHS Grand Visions Faculty Seed Grant “Analyzing the impact of steatosis and aberrant lipolysis on epigenetic modifications in cardiomyocytes”
- CEHS Kutscher Technology Innovation Grant “Thin Layer Chromatography - old methodology perfect for new students”
2023
- Layman Award 2023-2024 “The effect of LPCAT3 activation on cardiomyocyte metabolism during acute lipotoxicity”
- NPOD Project Leader 2022 “The role of LPCAT3 in pathogenesis of diabetic cardiomyopathy”
2022
- NPOD Program Seed Grant, September 2022, "The impact of obesity on satellite cells action in the skeletal muscle"
2020
- Vanderbilt Diabetes Center Discovery Program Grant, "In vivo 2H/13C flux analysis to assess LXR activation as a strategy to inhibit NASH pathogenesis"
2015
- National Science Centre in Poland PRELUDIUM 8 Grant, "Role of stearoyl-CoA desaturase 1 (SCD1) in regulation of adipose triglyceride lipase (ATGL) and lipolysis in cardiomyocytes"